Abstract
Much of nutrition research has been conventionally based on the use of simplistic in vitro systems or animal models, which have been extensively employed in an effort to better understand the relationships between diet and complex diseases as well as to evaluate food safety. Although these models have undeniably contributed to increase our mechanistic understanding of basic biological processes, they do not adequately model complex human physiopathological phenomena, creating concerns about the translatability to humans. During the last decade, extraordinary advancement in stem cell culturing, three-dimensional cell cultures, sequencing technologies, and computer science has occurred, which has originated a wealth of novel human-based and more physiologically relevant tools. These tools, also known as “new approach methodologies,” which comprise patient-derived organoids, organs-on-chip, multi-omics approach, along with computational models and analysis, represent innovative and exciting tools to forward nutrition research from a human-biology-oriented perspective. After considering some shortcomings of conventional in vitro and vivo approaches, here we describe the main novel available and emerging tools that are appropriate for designing a more human-relevant nutrition research. Our aim is to encourage discussion on the opportunity to explore innovative paths in nutrition research and to promote a paradigm-change toward a more human biology-focused approach to better understand human nutritional pathophysiology, to evaluate novel food products, and to develop more effective targeted preventive or therapeutic strategies while helping in reducing the number and replacing animals employed in nutrition research.
1 INTRODUCTION
The use of traditional cell culture and animal model methods in nutrition research throughout the 20th and 21st centuries has been fruitful in many cases, such as in increasing our knowledge of cellular signaling pathways, improving our understanding of fundamental mechanisms related to diet, and allowing for the mechanistic understanding of several human diseases as well as helping in finding new candidate drug targets. However, generalizing findings from these model systems to humans is a critical challenge. This problem is a crucial cause of the high proportion of failures encountered in moving candidate drugs from preclinical studies to clinical research and practice (Hartung, 2013; Marshall et al., 2023; Seyhan, 2019; Van Norman, 2019). An over-reliance on these models can significantly limit or even mislead our comprehension of human complex diseases and the effect of therapeutics, dietary active compounds, and dietary additives or toxins, as well as diet or nutritional interventions on human health.
The most widely used in vitro models for research, including nutrition research and food safety assessment, are based on monolayer, static, animal or human cell cultures or co-cultures, which are often not able to adequately model human/in vivo situations and mimic systemic responses.
On the other hand, despite the great genetic similarity between many laboratory animals and humans, animal models are often poor predictors of human health effects and pathophysiological processes (Attarwala, 2010; Dimitrov & White, 2016; Eastwood et al., 2010; Eddleston et al., 2016; Fratta et al., 1965; Gaukler et al., 2016; Greek & Rice, 2012; Hartung, 2009; Lauer et al., 2009; Mak et al., 2014; Martignoni et al., 2006; Mestas & Hughes, 2004; Pistollato et al., 2020; Seok et al., 2013; Toutain et al., 2010). Consequently, current drug development strategies based on animal modeling are increasingly called into question by the scientific community, recognizing the need to accelerate the growth of human-focused and human-relevant science and research both in toxicology/regulatory testing and in other fields (Archibald et al., 2018; Bailey, 2018; Bailey et al., 2015; Chandrasekera & Pippin, 2015; Greek et al., 2012; Hall, 2020; Herrmann et al., 2019; Horejs, 2021; Ingber, 2020; Marshall & Willett, 2018; Nuwer, 2022; Pistollato et al., 2015; Pound, 2020; Pound & Ritskes-Hoitinga, 2018; Seifirad & Haghpanah, 2019; Van Norman, 2019). In addition to scientific issues, there are increasing public concerns about laboratory animal suffering (EuropeanUnion, 2012; Strauss, 2018) and a commitment to explore alternative methods to replace laboratory animals in developing new drugs and products (Marshall et al., 2022; Neuhaus et al., 2022; Nuwer, 2022). However, conducting experiments on human subjects to test hypotheses and treatments related to human disease or to evaluate the safety of food or food additives may be difficult or impossible due to obvious ethical or practical issues.
During the last decade, extraordinary advancement in stem cell culturing, three-dimensional (3D) cell cultures, sequencing technologies, and computer science has occurred, which has originated a wealth of novel human-focused and more physiologically relevant tools. These tools, also known as “new approach methodologies” (NAMs), which comprise patient-derived organoids, organs-on-chip (OoC), multi-omics approach, computational models and analysis, along with interventional and observational studies on human subjects, are already yielding profuse and meaningful human-relevant data and promising results in several fields (Barrile et al., 2018; Ewart et al., 2022; Kamali et al., 2022; Passini et al., 2017; Pistollato et al., 2021, 2022; Ribeiro et al., 2022; Shi et al., 2017; Tovaglieri et al., 2019; Zheng et al., 2023).
After considering the most important shortcomings of conventional in vitro and vivo approaches (including conventional studies on human subjects), in this review, we describe the main available and emerging NAMs, taking into consideration their current and potential applications in the field of nutrition, in order to design a more human-oriented nutrition research (Supporting Information Table S1). Our aim is to encourage discussion on the opportunity to explore innovative paths in nutrition research and to promote a paradigm shift toward a more human biology-focused approach to better understand human nutritional pathophysiology, to support novel food assessment, and to develop more effective targeted therapeutic or preventive interventions, while help in reducing the number and replacing animals employed in nutrition research and food safety risk assessment.
2 SHORTCOMINGS OF TRADITIONAL MODELS
In vitro cell cultures, animal models, and human observational and intervention studies have traditionally been used and still are being used to study the effects of diet on health and the related physiological state. In this section, we discuss some of the most important limitations of conventional models with a particular focus on nutrition research. Some of the most important shortcomings of traditional in vitro and in vivo (including animal and human-based) models are visually depicted in Figure 1.

2.1 Traditional in vitro models
Conventional cell-based in vitro models, including HT-29 and Caco-2 cells, have been widely used to study intestinal barrier functions and host–microbiota–pathogens interactions (Hilgendorf et al., 2000), as well as the effects of dietary bioactive compounds (E. Y. Kim et al., 2008; Zhai et al., 2013). Human cell lines have been instrumental in gaining insights into the immune function, the effect of food bioactive compounds, and for studying the beneficial effects of probiotics (Yu et al., 2015); however, transformed cell lines of cancerous origin differ dramatically in their genetic repertoire and thus physiology (Pamies & Hartung, 2017).
These models also do not succeed in supporting the co-culture with commensal microbiota, which is very important for intestinal and systemic physiology (Lopez-Escalera & Wellejus, 2022). In contrast to the in vivo intestinal epithelium, Caco-2 cells do not use butyrate as an energy source, leading to an accumulation of butyrate and making it difficult to study the effects of short-chain fatty acids (SCFAs; Grouls et al., 2022).
Another limitation of these cell models is the absence of specialized cell types that can be differentiated from cell lines: The intestinal epithelium in vivo includes different cell types, such as enterocytes, stem cells, goblet cells, microfold cells, enteroendocrine cells, Paneth and tuft cells, which are not accurately represented.
A major drawback linked to the use of human primary cells and fresh tissues is the scarce availability of them. This is especially true for relatively inaccessible tissues such as the brain, heart, and kidneys. Furthermore, to obtain human specimens, invasive procedures such as biopsies or surgery are often required, and the samples frequently come from pathological findings, which may not be representative of human physiology.
Primary animal-derived cell and tissue cultures have been traditionally used in nutritional research (Naik et al., 2004; Sato & Clevers, 2013; Zietek et al., 2015); however, cells and tissues derived from nonhuman species might provide unreliable results for humans (Andersson et al., 2012; Dimitrov & White, 2016; Ginis et al., 2004; J. H. Kim et al., 2020).
Moreover, simplistic bi-dimensional (2D) cell cultures (e.g., Transwell models) could not reflect the human in vivo intestinal tissue morphology nor mimic other essential intestinal structures and functions, including villi formation, mucus production, and cytochrome P450 metabolism (Li et al., 2016).
Utilizing cells on a 2D monolayer and under static non-physiologic conditions could seriously impact the reliability of the results. In particular, the relevance of conventional static monolayer models may be reduced by the lack of physiological cues, such as the biochemical signals from other cell types, the physical stimuli from the 3D microenvironment, and the mechanical cues derived from movement (e.g., peristalsis) and the fluid fluxes (Pamies & Hartung, 2017). Static cultures have been proven to cause the inharmonious growth of cells and the accumulation of detrimental cellular metabolites, ultimately causing the death of intestinal epithelial cells and bacterial overgrowth (H. J. Kim et al., 2012).
The extensive use of animal-derived ingredients (e.g., serum, coating materials, growth factors) may cause additional problems, including experimental reproducibility issues and ethical concerns (Cassotta et al., 2022).
In addition, the great number of conditions to monitor and the numerous parameters to evaluate lead to artifacts as a consequence of cell culture procedures (Pamies & Hartung, 2017).
2.2 Animal models
Animal models (e.g., mouse model, dog, pig, etc.) have been traditionally used as a gold standard in nutrition research and for human safety studies (Baker, 2008). Some other nonmammalian models have been increasingly used in nutritional studies, such as birds (Baxter et al., 2018), zebrafish (Ulloa et al., 2011), insects (e.g., Drosophila melanogaster, Apis mellifera; Tonk-Rügen et al., 2022), and the worm Caenorhabditis elegans (Y. Wang et al., 2022). Animals have been used and still are being used for several studies including nutrient–nutrient interactions, assessment of bioavailability (Šimoliūnas et al., 2019) and the safety margins of nutrients (Baker, 2008), tolerance and safety tests of food and food additives (EFSA, 2008; Lin et al., 1992), host–diet–microbiota interactions (Rangan et al., 2019), studies of the impact of diet on health and disease, and the effect of potential therapeutic/dietary interventions (Yue Guo et al., 2018).
Among mammalian models, the mouse is the most commonly used model in nutrition research. Compared with other mammalian models, mice are small-sized, they have a lower cost of maintenance, their diet and environment can be effortlessly controlled, they are amenable to genetic manipulation, there are several genetic models already existing, and the use of inbred colonies reduces inter-individual variability (Nguyen et al., 2015). However, despite some similarities, there are important differences between human and rodent body size and lifespan, feeding patterns, dietary behavior, absorption, bioavailability and metabolism of dietary compounds or drugs, anatomy, and physiology of the digestive tract, as well as in microbiota composition and abundance.
For instance, it is important to note that mice and rats are coprophagic. Coprophagy influences the nutritional value of their diet by ensuring that some nutrients (e.g., vitamins and fatty acids) are not lost by defecation but reenter the intestine to be absorbed (Sakaguchi, 2003). Self-reinoculation with fecal microbes alters microbiota abundance and composition leading to a changed bile-acid profile in the mouse small intestine (Bogatyrev et al., 2020). Moreover, it has been shown that coprophagy prevention modifies microbial community structure, energy metabolism, neurochemistry, and cognitive behavior in Lasiopodomys brandtii, a small mammal (T. B. Bo et al., 2020).
Furthermore, mice and rats fed ad libitum and, especially, under dietary restrictions tend to consume cage bedding. The bedding type and the ability of mice to extract energy from the bedding may critically impact the results of several studies, including metabolic tests. An even greater impact is expected in the case of experiments that implicate caloric restriction (Gregor et al., 2020).
Considering that rodent models are extensively used to study the influences of diet and microbiota on human physiology and disease phenotype, as well as for compositional, spatial, temporal, and functional interrogation of the gut microbiota, coprophagy and bedding-eating may have profound implications on the reproducibility of the experimental results and their translatability to humans.
Much of nutrition research has been focused on the etiopathogenetic mechanisms of obesity, diabetes, and associated comorbidities (e.g., nonalcoholic fatty liver disease [NAFLD] and nonalcoholic steatohepatitis [NASH]) using rodent models, either genetically engineered or mutant mice or rats in which adiposity is induced through prolonged feeding on high-fat or high-density diets (Doulberis et al., 2020; Lutz & Woods, 2012). The choline- and methionine-deficient diet is one of the most researched models of NAFLD (Ibrahim et al., 2016).
However, despite the massive use of these rodent models, many mechanistic details of human metabolism remain poorly understood and therapeutic interventions for humans are limited and largely inadequate (B. Wang et al., 2014).
In genetic models, such as the leptin-deficient obese mouse (ob/ob mouse), due to the lack of action by the satiety factor leptin (or its associated receptor), the rodents spontaneously develop severe hyperphagia resulting in obesity and manifest some obesity-related conditions, including diabetes-like condition and hyperlipidemia.
Although such models are extensively used to study obesity-associated comorbidities, as well as to test novel drugs and/or dietary interventions, the disease manifestations in these models are the consequence of genetic mutations that do not mirror disease etiology in humans. One of the most striking characteristics of these models approximations is their monogenic inheritance pattern. Furthermore, these rodents have been inbred for many generations, and their genetics are homogeneous (B. Wang et al., 2014). This is in contrast to the etiology of complex human diseases, including human diabetes and NAFLD, which are not only polygenic but also multifactorial, with different genetic backgrounds.
Not surprisingly, important differences exist in the transcriptomic profile of the liver tissue, the way in which triglycerides accumulate within the liver, and the extent of hepatic fibrosis between human NAFLD and both genetic and dietary rodent models (Teufel et al., 2016).
Notably, although a methionine- and choline-deficient diet in mice reproduces several key clinical hallmarks of NASH, the metabolic profile induced by this dietary restriction is very dissimilar to human NASH, with observed weight loss rather than obesity, as well as a lack of insulin resistance and dyslipidemia (Ibrahim et al., 2016).
Compared with humans, rodents synthetize high quantities of cholesterol and bile acids, and they have faster clearance and lower levels of serum Low Density Lipoprotein (LDL) cholesterol (Straniero et al., 2020). Subsequently, since mice have very low levels of atherogenic lipoproteins, unlike humans, they do not develop significant atherogenic lesions when fed a Western-type high-fat, high-cholesterol diet (von Scheidt et al., 2017).
Definitely, we currently know much about rodent metabolism but still lack a comprehensive understanding of the mechanisms underlying glucose and lipid homeostasis in humans and the impact of chronic over-nutrition, as well as human obesity-associated diseases and responses to nutrition/therapeutic interventions (Lai et al., 2014).
Moreover, Musther et al. (2014) reported an extensive analysis of the published data to clarify the relationships between human and animal oral bioavailability. The lack of correlation in this extended dataset showed that animal bioavailability is not predictive of bioavailability in humans (Musther et al., 2014).
Animal models with considerable levels of genetic similitude to humans have been established to investigate the effects of food, dietary bioactive compounds, and drugs on digestion, absorption, and intestinal inflammation (Fois et al., 2019). These animal models can mimic certain aspects of the physiological processes occurring in vivo and may provide some mechanistic insights into the host–microbiota–diet interactions. However, there are several important differences between animal models and human systems, including the anatomy and physiology of the rodent and human gastrointestinal tract, which might be shaped by their diverging diets, nutrition patterns, metabolic demands, and body sizes. For instance, the human stomach is covered with a glandular mucosa that secretes gastric acid, whereas the mouse stomach is compartmentalized into two regions, a glandular gastric acid-secreting stomach and a nonglandular or fore-stomach that functions as a temporary site of food storage and digestion. The average proportion of the gut surface area and body surface area diverges substantially between mice and men over different sections of the gut (Casteleyn et al., 2010; Treuting et al., 2017). For example, the cecum is larger in the mouse, and it represents an important site for the fermentation of plant materials as well as for the production of vitamins B and K, which the mouse reabsorbs via coprophagy (Treuting et al., 2017).
These morphologic divergences suggest murine adaptation toward an increased capacity to extract nutrients from the significatively higher proportion of indigestible food components in their diet as compared with humans.
Mice and humans’ gastrointestinal tract also differs in histological features: For example, the colon of the mouse is composed of thin muscularis mucosae lacking an evident sub-mucosa, while the human colon is coated with a thicker mucosal wall. Another difference is the limited presence of transverse folds to the cecum and proximal colon in mice, whereas these folds are present in humans along the entire length of colon mucosa (Treuting et al., 2017). These differences in the gut micro-compartmentalization structure may result in important quantitative and qualitative divergences in the intestinal microbial communities. Indeed, only a limited percentage of the microbial genes are shared between mice and men (Hugenholtz & de Vos, 2018). In humans, three enterotypes can be detected, whereas only two can be identified in mice (Hildebrand et al., 2013; J. Wang et al., 2014), and 85% of the murine sequences concern species that have not been detected in humans (Ley et al., 2005).
There are also some crucial differences at the cellular level, for example, the distribution of mucin-producing goblet cells and Paneth cells that suggest differences in intestinal barrier functions and local immune responses.
In addition to the anatomy and histology, the gastrointestinal tract physiology of mice and humans are also different, for example, the intestinal transit time in mice is up to 10 times as fast as in humans. This is consistent with the overall metabolic rate, which is roughly seven times higher in mice, compared to humans (Treuting et al., 2017).
A study by Seok et al. (2013) revealed crucial differences in genomic inflammatory responses between humans and mice and among genes changed substantially in humans, and the murine orthologs are not far from random in matching their human counterparts (Seok et al., 2013). Considering that inflammation is an essential part of body defense/healing processes and is involved in several human conditions including obesity, diabetes, atherosclerosis, and cancer, relying on mouse models to study these conditions and their relations with nutrition may provide misleading results (Leist & Hartung, 2013).
Significant inter-species differences exist pertaining to vitamins, amino acids, lipids, and xenobiotics metabolism (Table 1).
| 1 | Lacking of biochemical signals from other cells and the extracellular matrix (Di Nardo et al., 2011; Pamies & Hartung, 2017) |
| 2 | Lacking of physical and structural stimuli from the three-dimensional microenvironment (Di Nardo et al., 2011; Pamies & Hartung, 2017) |
| 3 | Absence of mechanical stimuli derived from movement and the physicochemical fluxes originating from temperature, concentration, or momentum gradients (Di Nardo et al., 2011; Pamies & Hartung, 2017) |
| 4 | Metabolites and nutrients transport are limited by diffusion (Pamies & Hartung, 2017) |
| 5 | Difficulty in creating and maintaining controlled concentration gradients (Pamies & Hartung, 2017) |
| 6 | Impossibility of providing shear forces to maintain epithelial and endothelial polarization (Pamies & Hartung, 2017) |
| 7 | The microenvironment at the outer circumference of a well in a plate may differ from that at the center of the well (Hartung, 2007) |
| 8 | Lack of well-to-well connections with controlled flow to appropriately model organ– interactions (Pamies & Hartung, 2017) |
| 9 | |
| 10 |
Cancer origin of cell lines: drastic reduction of the expression of organotypic function and favoring of cell growth and division over other cell functions. Chromosomal aberrations and losses (Hartung, 2007); absence of specialized cell types that can be differentiated from cell lines (Pamies & Hartung, 2017) |
| 11 | Scarce availability of human-derived primary cells and fresh tissues; samples obtainable only through invasive procedures |
| 12 | Animal origin of cell cultures: may not reflect human physiopathology (Andersson et al., 2012; Dimitrov & White, 2016; Ginis et al., 2004; J. H. Kim et al., 2020) |
| 13 | The use of animal-derived and chemically undefined ingredients: reproducibility and contamination issues, ethical problems (Cassotta et al., 2022) |
Moreover, receptor activation and metabolic enzymes inducibility by chemical/food compounds substantially differ between animal models and humans (Hammer et al., 2021).
Zebrafish (Danio rerio) has emerged as a valuable model organism in nutritional research and for food toxicology assessment (Caro et al., 2016; Hou et al., 2023), offering unique advantages in studying various aspects of human health, including the intricate relationship between nutrition and the human microbiota. The transparency of zebrafish embryos and larvae facilitates real-time visualization, enabling researchers to track digestive processes and microbial interactions in vivo. These features have propelled zebrafish into the forefront of microbiota-related investigations, shedding light on how dietary components influence microbial colonization and subsequent host responses.
Although remarkable conservation exists between zebrafish and human genes associated with nutrient metabolism, gut development, and immune functions, zebrafish models possess inherent limitations that warrant careful consideration. Zebrafish have a simplified gut anatomy and lack certain complexities present in mammalian gastrointestinal systems, potentially limiting the direct translation of findings to humans. Differences in microbiota composition and metabolic pathways between zebrafish and humans necessitate cautious extrapolation of results. The microbial diversity and functionality of zebrafish gut flora differ substantially from humans, potentially affecting the applicability of zebrafish models in elucidating complex microbial interactions relevant to human health (Lu et al., 2021).
All these differences, together with the requirement to combine or alter animal models to suit precise nutrition research needs, make the extensive use of animal models in nutrition research extremely complex, difficult to validate and time-consuming, and the data resulting from using them still may not consistently translate into clinical outcomes. Table 2 outlines the key limitations linked with prevalent animal models frequently employed in nutrition research.
| Metabolic divergences |
|
| Differences in dietary behaviors |
|
| Differences in the gastrointestinal tract anatomy/histology/physiology |
|
| Differences in gut microbiota composition and abundance |
|
| Differences in immune response |
|
| Differences in disease etiopathology |
|
| General limitations |
|
2.3 Human studies
The human subject is the quintessential model for scientific research aimed at studying human physiopathology, including research in nutrition. Since research is carried out in humans, clinical nutrition studies can be promptly translated into public health messages. However, there are many challenges unique to the field, and it has always been difficult to study diseases and the effect of therapeutics and/or nutrition in humans in vivo (Hall, 2020). Although strong links between dietary habits and human health or disease are apparent from traditional epidemiology, the conclusions of extensive intervention studies exploring the causality of those relations have frequently proved unconvincing or have failed to establish causality, including nutrition interventional studies related to diseases (Bäcklund et al., 2023; Domínguez-López et al., 2020). This evident conflict may be related to the well-known difficulties in evaluating nutritional status and assessing habitual dietary intake that may result in confounding in observational and intervention epidemiology. Indeed, dietary intake is usually assessed by self-reported questionnaires, which have intrinsic limitations (Allison et al., 2015; S. Liang et al., 2022). Moreover, the background intake and status of a nutrient of interest usually are not assessed, and this can greatly influence the response being studied (Weaver & Miller, 2017). The most important drawbacks of traditional human studies are summarized in Table 3.
| 1 | Bias of self-reported dietary assessment, monitoring compliance with dietary protocols is difficult (Picó et al., 2019) |
| 2 | Difficulties in determining the biological effects of foods and their impact on health (Picó et al., 2019) |
| 3 | The background intake and status of a nutrient of interest (usually not assessed) can greatly influence the response being studied (Weaver & Miller, 2017) |
| 4 | Ethical constraints: navigating a complex maze of approvals (Weaver & Miller, 2017) and ethics limit the types of experiments and interventions that can be performed on human subjects, affecting the depth and scope of research |
| 5 | Potential for confounding factors: External factors like environmental influences or coexisting health conditions might confound research outcomes, complicating the interpretation of dietary effects |
| 6 | Interindividual variability: Humans exhibit wide variations in genetic makeup, metabolism, and responses to diet, complicating the generalization of findings |
| 7 | Difficulty in isolation: It is intricate to segregate the effects of individual nutrients or dietary components amidst the complexity of a person’s overall diet and lifestyle |
| 8 | Cost and duration: Long-term human studies are costly, time-consuming, and subject to compliance issues, affecting the feasibility of extensive research |
| 9 | Complexity of variables: Human studies involve numerous uncontrollable variables such as genetics, lifestyle, and dietary habits, making it challenging to isolate specific factors |
3 NAMs FOR NUTRITION RESEARCH
Recent advancements have brought up an astonishing array of research tools and approaches that are offering bold new ways to study human diseases and responses in a more human-relevant setting. These techniques include: i. human pluripotent stem cells (PSCs) and their differentiated derivatives (e.g., organoids), ii. dynamic cell cultures and OoC, iii. multi-“omics” technologies and approaches (e.g., transcriptomics, metabolomics, nutrigenomics) deriving from global analyses of biological samples by high-performing analytical approaches and databases, and iv. computational models.
Additionally, chemically defined nonanimal alternatives to animal-derived materials and reagents for in vitro experimentation are becoming more and more available, thus ameliorating the reproducibility of the experiments and solving both ethical and methodological problems associated with such materials and reagents (Cassotta et al., 2022).
Broad multi-scale and systems biology approaches are becoming crucially important as a result of the need to integrate the vast amount of incoming data. These approaches must consider all the different levels of biological complexity (including molecular, gene, and cellular level, organ/tissue, individual, and population level), thus allowing for the description of adverse outcome pathways (AOPs) as already envisaged for toxicology (Edwards et al., 2016) and proposed for several fields of biomedical research (Hogberg et al., 2022; Langley, 2014; Langley et al., 2015; Luettich et al., 2021).
3.1 Human-induced PSCs (hiPSCs) and organoids
Stem cells have the ability to self-renew and differentiate toward committed progenitor cells and mature specialized cells of multiple organ systems. They are generally categorized into embryonic stem cells found in the inner cell mass of the blastocyst, adult stem cells obtained from adult tissues, and iPSCs reprogrammed from adult somatic cells. iPSCs not only have the ability to undergo self-renewal and differentiation into any cell type of the body but can be also generated from quite easily accessible somatic cells, including skin-derived fibroblasts, blood-derived erythroblasts, or urine-derived epithelial cells (Raab et al., 2014), and their derivation does not involve destruction of embryos, thus avoiding ethical problems.
Moreover, there is no dependence on biopsy material derived from invasive endoscopic procedures. This enables the collection of source material from both healthy individuals and patients, allowing the study and the comparison between different genetic backgrounds. Subsequently, patient-specific iPSCs could provide unlimited disease-relevant cells in a personalized manner, serving as a valuable supply of previously inaccessible cell types, including cardiomyocytes (Karakikes et al., 2015), neurons (Alciati et al., 2022), intestinal (Grouls et al., 2022; Yoshida et al., 2021), hepatic (Inoue et al., 2020; Vallverdú et al., 2021) and pancreatic cells (Balboa et al., 2021; Choi et al., 2021; Genova et al., 2021).
Grouls et al. (2022) used iPSCs-derived intestinal epithelial cells, grown as a cell layer, to study the effects of the SCFAs butyrate, propionate, and acetate, on whole genome gene expression in the cells. Through this study, the authors have confirmed several known effects of SCFAs on intestinal cells, such as effects on immune responses and metabolism. The variations in metabolic pathways in the intestinal epithelial cell cultures in this study prove that there is a change in energy homeostasis, possibly linked to the use of SCFAs as an energy source by the iPSCs-derived intestinal epithelial cells mimicking in vivo intestinal tissues where microbiota-produced butyrate is a significant energy source (Grouls et al., 2022).
However, there are major challenges that need to be addressed to unleash the full potential of iPSCs. Although cost will likely decrease over time and several iPSC lines are commercially available, generating high-quality iPSCs is still expensive and time-consuming, and there is a lack of robust and reproducible iPSC differentiation protocols for the derivation of several cell types (Doss & Sachinidis, 2019).
Our ability to generate complex tissues in vitro from human stem cells continues to make rapid progress. Three-dimensional-cultured human organoids have become a compelling in vitro research tool that maintains genetic, phenotypic, developmental, and behavioral characteristics of in vivo organs, addressing some of the limitations of traditional culture systems. An organoid may be basically defined as a miniaturized organ that can be established from human stem cells in vitro, including iPSCs, and studied at the microscopic level. Today, we are able to generate functional cell types or organoids for most organs involved in nutrient regulation or metabolic organs, including (but not limited to) the stomach (McCracken et al., 2014; Seidlitz et al., 2021), intestine (Günther et al., 2022), liver (Guan et al., 2021; Thompson & Takebe, 2020), adipose tissue (W. Hu & Lazar, 2022; Mandl et al., 2022), skeletal muscle (J. H. Kim et al., 2022), pancreas (Hirshorn et al., 2021; Jiang et al., 2022), brain (Agboola et al., 2021), and heart (Xuan et al., 2022; Figure 2).

Human intestinal organoids (hIOs) have already been used to model nutrients transport physiology during digestion and drug uptake and metabolism (Foulke-Abel et al., 2016; Zietek et al., 2020) and epithelial barrier function (Holthaus et al., 2022; Leslie et al., 2015), as well as complex human diseases including celiac disease (Dieterich et al., 2020; Freire et al., 2019) and inflammatory bowel disease (IBD; Sarvestani et al., 2021). Pearce et al. (2020) have studied the effects of SCFAs on biomarkers of intestinal stem cells differentiation, barrier function, and epithelial defense in the gut using mouse and hIO models, showing that individual SCFAs are powerful stimulators of cellular gene expression and cell differentiation (Pearce et al., 2020). Intestinal stem cell-derived organoids from morbidly obese patients have been shown to preserve patient-specific obesity-related abnormalities in carbohydrate absorption and metabolism, providing an innovative preclinical platform to understand the physiopathology of obesity, and to analyze the heterogeneity of obesity mechanisms, as well as to identify novel therapeutic or nutritional interventions (Hasan et al., 2021). hIOs are also giving new opportunities to study the influence of diet on tumorigenesis. Toden et al. recently reported a strong chemoprotective role of flavan-3-ols (a commercial grape seed extract) in colorectal cancer by studying hIOs generated from colorectal cancer lesions as a preclinical model system (Toden et al., 2018). Deval et al. (2021) investigated molecular mechanisms underlying the risk of colorectal cancer from various carcinogens, including red/processed meat-derived carcinogens, by modeling exposure in normal human colon organoids (Devall et al., 2021).
Perlman et al. (2023) used human gastrointestinal organoids and organoid-derived monolayers to study the effect of malnutrition on the function of the gastrointestinal epithelium (Perlman et al., 2023).
By selectively limiting different macronutrient components of culture media, they were able to effectively culture and assess malnourished organoids. This study has shown that the malnourished media formulations and organoid culturing conditions are achievable and represent significant features of human malnutrition. This model raises several possibilities for nutrition research. For example, it would be possible to examine mechanistic underpinnings of common bacterial and viral gastrointestinal pathogens that behave differently in obese or malnourished patients. Moreover, the model could also be used to target high-impact nutritional supplements that could be provided in order to reduce morbidity.
hIOs integrated with human intestinal bacterial cells have been developed. These systems provide a microenvironment to model intestinal diet–microbiota–host interactions, giving new insights into the mechanisms by which microbiome dysbiosis and gut microbial metabolites may prevent or trigger diseases (M. B. Kim et al., 2022; Rubert et al., 2020).
Human midbrain organoids have been successfully used to study the association between aging and the gut microbiota-derived metabolite trimethylamine N-oxide (TMAO), derived from choline, betaine, phosphatidylcholine, and l-carnitine, which are abundant in some foods such as meat, egg yolks, and dairy products. Midbrain organoid treated with TMAO displayed aging-associated molecular changes, including increased senescence marker expression and epigenetic alterations. Moreover, TMAO-treated midbrain organoids have shown neurodegeneration phenotypes, including loss of dopaminergic neurons, neuromelanin accumulation, α-synuclein, and Tau proteins modifications. These results have suggested a role of TMAO in the aging and pathogenesis of the midbrain, providing insight into how nutrition or intestinal dysfunction may increase the risk of neurodegenerative diseases such as Parkinson’s.
Since organoids recapitulate human development, they can be used to observe cellular responses to genetic or environmental perturbations at different stages of cellular development and differentiation. For example, Adams et al. have recently used human cerebral cortical organoids to study the impact of alcohol exposure on neurogenesis. Alcohol-exposed cortical organoids showed compromised cell growth and viability, characteristic alterations in their epigenomic and gene expression profiles in regions crucial to neurodevelopment, and underwent dysfunctional neuronal network formation, mimicking the developmental neuropathology of prenatal alcohol exposure (Adams et al., 2022).
The opportunity to genetically modify stem cells that are employed to establish 3D complex in vitro models will be highly useful in defining which genes function in which cells to influence phenotype. Advances in gene editing by TALEN and CRISPR technologies allow us to test both gain and loss of function for specific genes and organs (De Masi et al., 2020).
Despite these model systems being very promising, they have still some critical limitations. An important limitation of organoid-based models is the lack of a vascular system. In vivo, tissues are permeated with complex vascular networks to allow the exchange of oxygen, transport of nutrients, waste, metabolites, growth factors, hormones, and so forth, whereas in vitro, the microenvironment of organoids is still incomplete, making large sizes and long-term cultures difficult to maintain. Moreover, current organoids lack some cellular populations found in the native organ including immune, nerve, and mesenchymal cells, not being able to completely simulate the in vivo microenvironment of several tissues or diseases. Another important limitation is the lack of the physiological process of mutual communication between different organs, so they cannot reflect systemic responses.
However, the field of applications for organoids is rapidly developing, and there is progress toward more complex and sophisticated organoid-based model systems. For example, co-culture with mesenchymal stem cells, endothelial cells, and specific growth factors on innovative 3D substrate matrix, as well as 3D bioprinting, allow to generate latest generation vascularized organoids (Dellaquila et al., 2021; Ren et al., 2021; S. Zhang et al., 2021). Co-cultures with different cell types, including immune and nerve cells (Schreurs et al., 2021; Tominaga et al., 2022; Tsuruta et al., 2022), the combination of multiple organoids or the integration of organoids with missing cell types or primary tissue explants (assembloids; Kanton & Paşca, 2022; Shek et al., 2021), as well as approaches to replicate the complex dynamic tissue environment encompassing continuously flowing fluid systems or to replicate multi-organ interactions have been established (Park et al., 2019), for example, fluidic bioreactors or OoC/microphysiological systems (MPS). An overview of the main current or potential applications of iPSCs and iPSCs-derived organoids in nutrition research, along with their most important limitations and the possible ways to overcome these limitations, is presented in Table 4.
| New approach methodology (NAM) | Examples of applications to nutrition research | Limitations | Possible ways to overcome limitations |
| iPSCs-derived organoids (gastro-intestinal, pancreatic, hepatic, cardiac, etc.) |
– Studying developmental effects of nutrients – Investigating relevant pathogenetic mechanisms of diet-related diseases: deciphering risk factors – Exploring genetic and sex differences, gene-environment interaction – Identifying food bioactive compounds; high-throughput screening for compounds that can inhibit or ameliorate diet-related human diseases – Personalized medicine – Studying the host–diet–microbiota relations |
Generating high-quality iPSCs is expensive and time-consuming |
Cost will likely decrease over time; iPSC lines are commercially available; generating more characterized and sophisticated models of organoids |
| Lack of robust and reproducible iPSC differentiation protocols for derivation of the several cell types | |||
| Organoids represent an early stage of embryonic development, while many human diseases are late-onset conditions |
– Inducing an overexpression of aging-related genes (such as progerin) to recapitualate aging and late-onset diseases – Direct conversion of aging donors’ fibroblasts into specialized cells can help retain aging-related transcriptional signatures (Torrens-Mas et al., 2021) |
||
| Organoids may not recapitulate diseases progression | Combine with patients’ in vivo and postmortem studies | ||
| Gut-organoids: Basal-out structure may cause difficulties in research on the intestinal apical side | Utilizing apical-out organoids (Co et al., 2021) | ||
|
Intestinal organoids: – Lack of complex mesenchymal heterogeneity, architecture, vasculature, neuronal connections and interaction with immune cells, and the intestinal microbial flora – Lack of adequate oxygen and nutrient supply and the accumulation of metabolic waste |
– Co-culturing with mesenchymal, endothelial, immune, glial, and microbial cells, adding vasculature (Wörsdörfer et al., 2020); – Combining with fluidic technologies (Park et al., 2019) |
||
| Random and uncontrolled nature of organoids’ growth | Better characterization of the models and standardization of culture protocols |
3.2 Multi-compartmental modular bioreactors (MCMBs)
In an effort to create and optimize more human-relevant in vitro models and increase predictive capacity, a wide range of in vitro dynamic fluidic culture systems have been developed. Unlike static culture conditions, the use of bioreactors carries the potential to achieve a more tissue- or organ-specific dynamic culture by providing mechanical stimulation, better nutrient transport, oxygenation, and waste removal.
The MCMB system consists of modular cell culture chambers made of transparent, flexible bio-compatible silicon polymer, with shape and dimensions similar to the 24-MultiWells. The modular chambers can be connected together to a hydraulic circuit that perfuses the culture medium with a pump, in series or in parallel, in order to allow cell–cell cross-talk or to model in vivo systemic responses, using allometric design principles (Guzzardi et al., 2011; Sbrana & Ahluwalia, 2012; Schmelzer & Gerlach, 2016). The membrane bioreactor is a double-flow system suited to model physiological barriers, which associates a transwell-like structure with fluidic flow and multi-compartmental systems. A porous membrane, whose features and permeability may differ according to research needs, separates the bioreactor into two individual chambers for dynamic in vitro investigations of nutrients or drug diffusion through physiological barriers, including intestinal barrier (Cacopardo et al., 2019; Giusti et al., 2014; Lombardo et al., 2021). These platforms can be employed to assess the passage and biodistribution of orally administered compounds, with high predictability and reproducibility (Figure 3).

Colombo et al. (2019) used MCMBs to develop a model of human gastrointestinal tract in order to evaluate the effects of dietary methylglyoxal, an extremely reactive α-oxoaldehyde responsible for the formation of advanced glycation end-products associated with several chronic diseases. They found a new role of gastric cells in the metabolization of methylglyoxal and other toxic compounds (Colombo et al., 2019), underscoring the importance of these advanced in vitro systems as high-throughput compound screening tools in food analysis, drug discovery, and toxicity tests.
Marrella et al. (2020) have developed an in vitro perfused model of the small intestinal barrier utilizing a 3D human reconstructed intestinal epithelium incorporated into a fluidic bioreactor mimicking the in vivo stimuli of the intestinal environment. This platform could be used as an innovative model of the small intestinal barrier to study the passage of molecules in both healthy and pathological conditions, as well as to test the effects of dietary compounds or therapeutics on intestinal tissue barrier function (Marrella et al., 2020).
Multi-organ platforms supporting the fluidic connection of the gut compartment and other organs (such as liver, adipose tissue, and kidney) can make these models even more predictive on Absorbtion, Distribution, Metabolism, Excretion (ADME), pharmacokinetic assays, and in the study of human disease and the effect of dietary bioactive compounds.
Connected cultures of hepatocytes, adipose tissue, and endothelial cells in MCMBs have already been successfully used to investigate the regulation of systemic glucose and lipid metabolism in vitro. Metabolite dynamics have been analyzed in four different physiological and pathological conditions including fasting and the postabsorptive state and the postprandial state in type 1 and type 2 diabetes, respectively. The results demonstrated that connected cultures can recapitulate complex physiopathological systemic processes including the key features of human metabolism and systemic inflammation in the presence of nutritional overload (Iori et al., 2012; Vinci et al., 2012).
3.3 OoCs and MPS
OoCs are microfluidic cell culture platforms in which cells are cultured in engineered devices that mimic the key aspects of multicellular architectures, tissue–tissue interfaces, physicochemical microenvironments, dynamic, flow, and gradients observed in the human body. OoCs can be considered miniaturized versions of multi-compartmental bioreactors.
A wide range of human tissues and organs have been modeled, including stomach (K. K. Lee et al., 2018), gut (Bein et al., 2018), immune cells and organs (Morsink et al., 2020), liver (Deng et al., 2019), pancreas (Venis et al., 2021; Yin et al., 2022), fat (Pope et al., 2020), heart (Criscione et al., 2023), brain (Nandi et al., 2022), and blood–brain barrier (Peng et al., 2022).
Microfluidic intestine-on-chip models have emerged as innovative platforms to study intestinal functions through the incorporation of different cell types into the system (Donkers et al., 2021; Marrero et al., 2021). The precise control of differentiation conditions offered by microfluidic perfusion combined with advanced cell substrates has great potential for improving stem cell differentiation and maturation to faithfully mimic in vivo human region-specific intestinal architecture and cellular and molecular composition (Siwczak et al., 2021).
Human colon-on-chip provides valuable innovative models to study colonic physiopathology and the effect of drugs or nutraceuticals in a complex yet controllable manner. A typical colon-on-chip model includes two parallel channels separated by an extracellular-matrix-coated porous membrane, allowing cell–cell communications between epithelium and vasculature. Vacuum channels along with the cell culture channels enable the application of cyclic stretch to simulate intestinal peristalsis (Morelli et al., 2023). In more sophisticated gut-on-chip models, human intestinal epithelium, capillary endothelium, immune cells, and the microbiota, functionally coexist and interact with each other (Figure 4).

Although the applications of gut-on-chip models are still at the initial phases of development, they hold great potential for exploring the interactions between host, microbiome, and nutrition (Garcia-Gutierrez & Cotter, 2022; D. Liang et al., 2022; Siwczak et al., 2021; Wu et al., 2023).
Fluidic flow and peristalsis have been found to be a critical factor in modulating active host–microbiota interplay (H. J. Kim et al., 2012).
Human gut-on-chip models must accurately reproduce in vivo oxygen concentration gradients to mirror metabolism, gene expression, and host–microbiome interactions (Chikina & Matic Vignjevic, 2021). While Grant et al. (2022) described a simple strategy to obtain physiologically relevant oxygen tension in a two-channel human small intestine-on-a-chip, Liu et al. (2023) recently proposed a gut-on-a-chip model simulating an in vivo-like controllable oxygen gradients across the intestinal epithelium to study the effect of Bifidobacterium bifidum supplementation on IBD. This probiotic supplement, which has already been shown to aid in the prevention, easing, and treatment of IBD in humans, has been validated to contribute to the integrity of the intestinal epithelial barrier, by preventing epithelial barrier disruption and promoting the repair of damaged intestinal epithelial cell monolayers.
A modular, microfluidics-based gut-on-chip model has been recently used to investigate the effects of SCFAs released by probiotic Lactobacillus rhamnosus on colorectal cancer (Greenhalgh et al., 2019). In particular, it has been shown that SCFAs and lactate production were altered by a simulated high-fiber intake, compared to a reference medium containing only simple sugars. The simulated high-fiber diet increased the expression of oncogenes and proinflammatory signaling in the absence of L. rhamnosus supplementation, while in the presence of the probiotics, both gene clusters were shown to be considerably downregulated and correlated with a decreased cell proliferation rate of primary colorectal cancer cells. This study demonstrates the ability of gut-on-chip systems to accurately dissect the distinct aspects of the microbiota–host interaction at the metabolic level.
The microbiota can also be obtained from distinct patients or food consumers, enabling potentially valuable studies related to personalized diagnostics and treatments (Garcia-Gutierrez & Cotter, 2022).
K. W. Lee et al. (2023) recently suggested the development of a novel research design using a synthetic bacterial community in gut-on-chip to analyze bacteria–bacteria interactions and the diet–microbiota relationship (K. W. Lee et al., 2023). This approach will allow to discover under-recognized functionalities of food substances and investigate metabolic interactions in the gut microbiota affected by dietary patterns. Arranging synthetic gut microflora using various bacterial species and tailoring these models to match individual gut microbiome compositions could offer support in creating personalized food items and nutraceuticals.
Gut-on-a-chip models may prove particularly useful for food safety assessment (S. H. Lee et al., 2019) as well as for the evaluation of bioactive components and novel food product development (Wu et al., 2023). A gut-on-chip model coupled to an ultra-performance liquid chromatography quadrupole time-of-flight mass spectrometer has been recently used for alternated analytical evaluations of the apical and basolateral concentrations of ergotamine epimers, natural-occurring toxins in food. This study showed for the first time, epimer-specific ergotamine transport across gut epithelium (Santbergen et al., 2020).
Recently, a flexible and reconfigurable microfluidic chip has been successfully tested for human immune cell culture, activation, and quantification of inflammatory cytokine secretion with the aim of assessing dietary supplements for anti-inflammatory properties. The chip included three fluidic layers for perfusion, immune cell culture, and cytokine capture and quantification. The perfusing media were separated from the cell culture by utilizing a biomimetic membrane to simulate the intestinal epithelial layer. A human peripheral blood monocytic cell line and its induced macrophages were employed as a model of immune-responsive cells. The cells were consecutively stimulated by lipopolysaccharides and two well-known inflammasome-modulating dietary supplements, that is, curcumin and docosahexaenoic acid (DHA). Both curcumin and DHA have shown anti-inflammatory effects by downregulating the secretion of Tumor Necrsis Factor (TNF)α, Interleukin (IL)-6, IL-1β, and IL-10, demonstrating the potential of this system for the screening of anti-inflammatory/inflammatory properties of supplements or dietary compounds (Ramadan et al., 2022).
While fat-on-chip models are very promising to study obesity and its metabolic comorbidities (McCarthy et al., 2020), liver-on-chip technology is providing long-term (>1 month) in vitro cultures of primary human hepatocytes, Kupffer, and stellate cells in 3D constructs that capture key NAFLD/NASH hallmarks such as intracellular fat accumulation, inflammation, and fibrosis. It could enable the specific mechanistic effects of compounds (including dietary-derived compounds) to be teased out and for models to be easily manipulated to suit research needs (Kostrzewski et al., 2020). This is very important considering the scarce clinical relevance of animal models of obesity and obesity-related pathology, including NAFLD and NASH.
While each OoC is a limited representation of the single organ it mimics, it can be employed in connection with other OoC systems. As the human digestive system depends on the full functioning of all its organs involved, in the same manner, it is possible to establish a complex network of microfluidic systems reflecting the whole path of a specific administered substance from one organ to the next. With such tools, it would become feasible to determine in vitro the influences of a dietary bioactive compound or nutraceutical after its ingestion. Miller and Shuler (2016) described a human “body-on-a-chip” MPS including 13 multiple chambers representing different organs (Miller & Shuler, 2016). The connection of different OoCs on multi-organs-on-a-chip platforms, namely, MPS, mimicking the interface and communication among barriers, parenchymal tissues, and the systemic circulation, has already offered new chances to study the absorption, distribution, metabolism, and bioactivity of nutritional compounds in vitro with unprecedented physiological accuracy (Picollet-D’hahan et al., 2021).
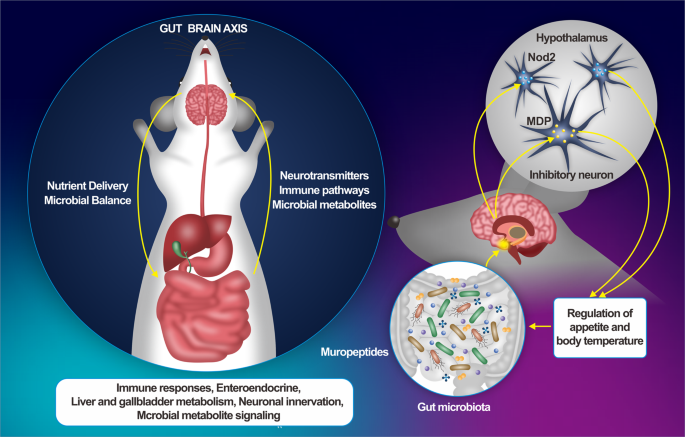
The complex cross-talk between the gut microbiota and the host’s immune system unavoidably influences the function of other organs, creating an “axis” between them. MPS are being developed to recapitulate the gut–microbiota–organ axis (Yuxi Guo et al., 2023). Since receptors for hormones produced by intestinal enteroendocrine cells have been identified in the gut and distinct brain regions, and considering that these hormones have a pivotal role as signaling systems in satiation mechanisms (D’Agostino & Small, 2012; Neary & Batterham, 2010), MPS could provide, for example, a robust platform for evaluating the potential of macronutrients as modulators of the microbiota–gut–brain axis, in order to design functional foods based in bioactive compounds, which may offer effective, alternative treatments for obesity and/or associated metabolic diseases (Pizarroso et al., 2021).
Slaughter et al. (2021) developed an MPS consisting of human hepatocytes and adipose tissue chambers suitable for modeling the metabolic factors that contribute to NAFLD development and progression and evaluation of therapeutic compounds (Slaughter et al., 2021).
By combining hiPSC and OoC technologies, in vitro models can now be established that integrate the genetic background of complex diseases, the different interacting cell types involved in a specific disease process, and the modulating environmental factors such as diet-derived compounds (e.g., gluten) and the gut microbiome (Moerkens et al., 2019; Palasantzas et al., 2023).
Janssen et al. (2023) recently proposed an engineered gut–immune–skin axis multi-organ-on-chip to better evaluate food allergen sensitization and advance mechanistic insight into the cross-talk between the gut, skin, and immune cells essential for food proteins allergy prediction (Janssen et al., 2023).
Although OoC model systems have great potential to expand our understanding of complex diseases etiology and accelerate the development of novel therapies, nutritional interventions, and preventive measures, there are still some limitations to overcome. While advanced microfluidic OoCs models can simulate various human phenotypes and organ responses, they currently fall short of fully replicating all aspects of organ tissues. For instance, in current gut-on-chip models, the four-layered intestinal wall is not entirely mimicked, yet all these layers are crucial for accurately simulating certain disorders that involve coordinated actions among them. To adequately replicate these disorders, it is imperative to incorporate all four layers and their defining characteristics into intestinal-organ-on-chips. Hence, enhancing these models by including additional components becomes crucial for achieving more sophisticated applications in the future.
Moreover, developing and maintaining OoC models can be technically challenging and labor-intensive, demanding expertise in microfabrication, engineering, and biology. In addition, the upfront expenses associated with establishing these systems can be substantial (Candarlioglu et al., 2022).
An outline of the current or prospective uses of MCMBs, OoC, and MPS in nutrition research, their primary limitations, and potential strategies for addressing these constraints is depicted in Table 5.
| NAM | Examples of applications to nutrition research | Limitations | Possible ways to overcome limitations |
| MCMBs, OoC, MPS (e.g., gut-on-chip, liver-on-chip, fat-on-chip, gut-microbiota-liver-brain-on-a-chip, etc.) |
– To assess the passage and biodistribution of orally administered compounds – To study the passage of molecules in both healthy and pathological conditions – To test the effects of dietary compounds or therapeutics on intestinal tissue barrier function – To perform pharmacokinetic assays – Studying human disease and the effect of dietary bioactive compounds – Investigating the regulation of systemic glucose and lipid metabolism in vitro |
Limited lifespan of certain types of cells, for example, endothelial cells |
– Extensive characterization of bioreactors, OoC and multi-organs-on-chip – Perfecting culture methods and chambers or chip construction materials (Ashammakhi et al., 2020) |
| Endocrine and immunological functions in gut-on-a-chip models are still poorly represented | Implementing immunoresponsive gut-on-chip systems (De Gregorio et al., 2022) | ||
| The four-layered intestinal wall is not entirely mimicked in gut-on-chip | Incorporating all four layers and their defining characteristics into intestinal-organ-on-chips | ||
| Developing and maintaining these models can be technically challenging and labor-intensive | Promoting multidisciplinary collaborations | ||
| The upfront expenses associated with establishing organ-on-a-chip systems can be substantial | Considering long-term savings since failed animal experiments or failed clinical trials may be avoided |
3.4 Omics and multi-omics approaches
Novel high-throughput/high-content techniques, together with the evolution of new computational models and statistical tools have led to the opportunity to analyze and filter great amounts of data and details at the molecular level. Advances in next-generation sequencing (NGS), mass-spectrometry, nuclear magnetic resonance, high-throughput platforms, and bioinformatic tools allowed the simultaneous comprehensive study of large numbers of genes (genomics), RNA (transcriptomics), metabolites (metabolomics), proteins (proteomics), epigenetic factors (epigenomics), lipids (lipidomics), and human microbiota (microbiomics) with the ability of merging different types of “omics” data (“multi-omics” or “system biology”).
A primary objective within nutritional research involves elucidating nutrition’s role in metabolic regulation and its impact on overall health. Conventionally, the correlation between nutrition and health has been explained by the body’s energetic and structural needs fulfilled by essential nutrients. However, beyond essential nutrients, foods contain numerous secondary bioactive compounds that play a role in preventing and potentially treating various chronic diseases. Epidemiological studies have established links between nutrition and the incidence of conditions such as type 2 diabetes (Baleato et al., 2022; Popkin, 2015), cardiovascular diseases (Becerra-Tomás et al., 2019; Papier et al., 2023), cancer (Bouvard et al., 2015; Key et al., 2020), and neurodegenerative disorders. Despite these associations, understanding the precise components and mechanisms underlying their beneficial or detrimental effects remains incomplete.
Modern high-throughput omics approaches have revolutionized the exploration of connections between dietary intake and health outcomes at a molecular level, accelerating the identification of molecular events associated with nutritional effects on health or diet-related diseases (Trujillo et al., 2006).
Nutrigenetics, a field identifying gene variants influencing responses to nutrients and their connection to disease states, has gained prominence. NGS enables rapid, cost-effective reading of vast quantities of DNA or RNA fragments simultaneously, facilitating the identification of genetic variations. Integrating genetic polymorphisms into nutritional epidemiological studies has addressed inherent limitations, such as genetic variability impacting nutrient absorption, metabolism, or elimination (El-Sohemy, 2007; Gomez-Delgado et al., 2014).
An example highlighting the synergy between nutrigenomics, NGS technologies, and precise dietary factors involves the investigation of coffee’s impact on heart disease (Cornelis et al., 2006). While studies on coffee’s effects yielded conflicting results—suggesting risk reduction, neutrality, or increased risk (Ordovas & Corella, 2004)—caffeinated coffee was found to elevate heart attack risk in individuals with a gene variant linked to “slow” caffeine metabolism, with no effect on “fast” caffeine metabolizers (Cornelis & El-Sohemy, 2007).
NGS and omics-based applications in nutrigenetics offer crucial insights that will aid clinicians in tailoring personalized nutrition plans for individuals.
Transcriptomics studies, facilitated by technologies like real-time Polymerase Chain Reacion (PCR) and RNA sequencing, provide comprehensive views of intracellular RNA expression under specific nutritional conditions (Tachibana, 2015; Zhao et al., 2014). Transcriptomics has been pivotal in investigating the effects of compounds like anthocyanins on obesity-associated gene expression in human adipocytes (Tsuda et al., 2006) and in studying gene expression changes in blood to explore nutritional influences in human intervention studies (van Erk et al., 2006).
Nutriproteomics, a branch of proteomics within nutrition science, utilizes advanced proteomic technologies to comprehensively analyze variations in protein expression and function. It investigates how food components interact with proteins within the body, potentially inducing post-translational modifications that alter their original functions. Understanding and characterizing these modifications can provide deeper insights into the interplay between bioactive dietary components and diseases related to diet (Ganesh & Hettiarachchy, 2012; Schweigert, 2007). For instance, nutriproteomics holds promise in uncovering potential connections between food antigens and autoimmune disorders (Vojdani et al., 2020).
The integration of different “omics” layers can be harnessed to establish a more realistic and multi-tiered view of biological systems and complex diseases. They have revolutionized the way human diseases are studied, providing a holistic understanding of basic functional mechanisms, and interactive molecular regulatory information flow for disease susceptibility, risk, and traits (Chen et al., 2020; Lloyd-Price et al., 2019; Q. Zhang et al., 2022), also allowing for patient stratification for treatment and response (C. Hu & Jia, 2021; Mars et al., 2020; Figure 5).

Leonard et al. (2020) leveraged multi-omics analysis to show the influence of genetic and environmental risk factors on developing gut microbiota in infants at risk of celiac disease. Among their noteworthy findings, the authors found that cesarean section delivery was associated with a decreased abundance of specific gut bacteria and folate biosynthesis pathway and with an increased abundance of specific microbial metabolites, linked to alterations that are implicated in immune system dysfunction and inflammatory conditions.
Moreover, multi-omics approaches can provide unprecedented insights into species-specific mechanisms of disease pathogenesis. For example, by combining microfluidic in vitro culture technologies (colon-on-chip) and multi-omics approach, Tovaglieri et al. (2019) explained the mechanisms underlying the increased susceptibility of humans to enterohemorrhagic Escherichia coli (EHEC) infection, compared to mice. In particular, they discovered four human-specific microbiome metabolites that mediate this effect, preferentially inducing the expression of flagellin, a bacterial protein linked with the motility of EHEC and enhanced epithelial damage.
Applications of the multi-omics approaches also accelerated molecular nutrition understanding. Consequently, nutrigenomics has emerged as an interdisciplinary research field in nutrition science that aims at clarifying how nutrition can affect human health (Sales et al., 2014). Novel high-throughput “omics” approaches are helping to understand the links between dietary exposure and health at the molecular level, from the perspective of personalized nutrition (Aruoma et al., 2019; Valdés et al., 2017). Multi-omics approaches are very promising for personalized disease-risk stratification, for example, they are being employed to study the associations between metabolites, novel biomarkers of cancer, and dietary patterns in the context of colon cancer prevention (Amani Mohammad et al., 2022).
The combination of in vitro complex technologies, for example, intestinal organoids, and multi-omics approaches, principally metabolomics and comprehensive lipidomics, could allow for new insights into the mechanisms through which nutrient–gene or microbiome–gut interplay may impact the intestinal stem cell niche. This could help researchers to understand the role of microorganisms and gut microbiome-metabolites in personalized nutrition as well as the initiation, progression, and prevention of diet-related diseases (Rubert et al., 2020).
The integrated use of several “omics” approaches is also enabling the discovery of new biomarkers correlated with specific dietary intake or food, significantly facilitating human nutritional studies. To process the assessment of a person’s dietary intake, and thus elucidate the possible relationships between diet and disease, relevant and accurate dietary assessment methods are critical. Dietary biomarkers have emerged as a complementary tool to the conventional methods in nutrition research, and in the few past years, metabolomics has arisen as a high-performance and sensitive approach for evaluating metabolite profiles resulting from specific dietary intake, as well as for the identification of new dietary biomarkers (Collins et al., 2019).
Dietary biomarkers may allow to accurately and objectively evaluate food intake by measuring urine/fecal/blood metabolites thus avoiding the biases that self-reporting of food consumption may introduce. There are various metabolomics and proteomics studies that have identified candidate biomarkers for distinct dietary behaviors and for several kinds of foods, including vegetables, fruits, and meat. Some studies have also described metabolites linked to particular dietary patterns (Coras et al., 2020), such as high-fat diet and Mediterranean or Western diets. The discovery of food biomarkers is in progress; however, several results are just associations, and they lack the desired validation including dose–response studies.
The application of biomarkers in nutrition research will be very important in the near future to improve the assessment of dietary intake, to classify individuals into consumers/nonconsumers of specific foods, or into dietary patterns. Food intake biomarkers can also play a role in assessing compliance with dietary interventions, as well as in providing information on interindividual variations in dietary responses (McNamara & Brennan, 2020).
The progress of high-throughput NGS technologies has enabled omics investigation at single-cell resolution. For example, single-cell transcriptomics has emerged as an innovative approach to decompose tissues into different cell types for the study of transcriptional profiles of individual cells. The expanding role of single-cell RNA sequencing in nutrition research may decipher the variation of cell specifications for diet interventions and comparing healthy and disease-associated tissues at single-cell resolution, with an ultimate goal to enhance our understanding of the links between diet and health. Using single-cell transcriptome analysis of epithelial cells from human ileum, colon, and rectum, Wang et al. (2020) revealed different nutrient-absorption preferences in the human large and small intestine, providing a great potential for further characterization of human intestine cell constitution and functions. By comparing the transcriptomes of human and mouse ileum epithelial cells, the authors also found dissimilar gene expression patterns in human and mouse ileum (Wang et al., 2020).
In recent years, with the advancement of high-resolution and accurate mass spectrometry, also metabolomics entered a new “era,” promoting its broader applications in nutrition research. Li et al. (2022) recently discussed the emerging roles of next-generation metabolomics, including single-cell metabolomics, in advancing our understanding of critical care nutrition, such as metabolic mechanisms of nutritional therapies, nutritional deficiency risk evaluation, and novel nutrition target identification (Li, Tong, Chen, Sun, & Wang, 2022).
The application of the omics approach to single cells has developed into a new and exciting field of research where multi-omic layers of DNA, RNA, proteins, methylated DNA, or metabolites can be simultaneously profiled in the same cell to analyze the causal mechanisms. Sequencing of bulk tissues is being replaced by single-cell multi-omics, where physiopathological processes can be dissected at single-cell resolution, enhancing our understanding of the cellular characteristics and population architectures of heterogeneous tissues. This can provide snapshots of the relationship between these multi-omic molecular layers and the complexity encompassing different levels of biological organization. Although single-cell omics has a broad array of applications in biomedical research to compare healthy and disease-associated tissues, this approach is still in its infancy in nutrition science and food toxicology (X. Wang et al., 2024).
Despite the omics and multi-omics approaches bringing great opportunities for nutrition research, there still remain several challenges that require attention to realize the full potential of combining high-throughput data obtained from different molecular layers. These challenges comprise the heterogeneity among omics technologies, the handling of missing values, the difficulty of interpreting multilayered systems models, and the issues pertaining data annotation, storage, and computational resources (Tarazona et al., 2021).
3.5 Computational models
Computational models (also known as in silico models) combine mathematics and computer science methods. They include, but are not limited to, machine learning (ML), artificial intelligence (AI), and quantitative structure–activity relationships (QSAR) models. The use of computational modeling and simulation has expanded in many fields, including nutrition research. A great variety of computational modeling approaches have been applied to broad-ranging biological levels of organization, from molecules to human organisms. The processes that can be modeled comprise molecular interactions, signaling and metabolic pathways, cell growth, anatomical structures, and physiological processes. Moreover, computational tools can support complex in vitro models for system-level understanding of complex processes and/or for human-relevant in vitro–in vivo extrapolations (Algharably et al., 2022; Andreoni et al., 2014; Casas et al., 2022). Computational modeling approaches are essential for quantitatively analyzing OoC systems and predicting their complex responses (Sung, 2022).
Therefore, computational approaches vary broadly with application.
Nutritional science is currently undergoing a data explosion as a growing number of studies are integrating methods from genomics, transcriptomics, proteomics, metabolomics, and so forth. Accordingly, an important challenge for nutrition research is to connect high-dimensional datasets that are collected at vastly different spatial, temporal, and dimensionality scales. Computational modeling provides a means to formulate novel solutions to such systems-level problems, allowing to analyze/integrate great amounts of multi-dimensional datasets, and/or massively interacting systems, such as the relationship between nutrients, human microbiome, metabolism, immune response, health, and disease (Allison et al., 2015; Verma et al., 2016).
In silico tools have been utilized to model the digestion and absorption of several drug molecules, and they can be applied also to nutrients, including lipophilic micronutrients, taking into consideration the parameters as human digestion conditions or food matrix (Marze, 2014). Computational modeling of digestion is a promising tool to advance our understanding of the interactions between diet and the comprehensive functioning of the human gastrointestinal tract and post-absorptive processes. In the same way as drugs, the dose and timing of nutrients entering the bloodstream during digestion have significant metabolic repercussions. Examples comprise both harmful and beneficial consequences, for instance, the enhanced risk of type 2 diabetes for diets with high glycemic index (Bhupathiraju et al., 2014), or the stimulation of muscle proteins synthesis over a threshold of leucine in the bloodstream (Rieu et al., 2006). Computational models can be employed to address the variety of the processes that take place during digestion and could be very important for predictive purposes. This has already occurred in the field of pharmacology, where the concept of “in silico clinical trials” has developed as a novel approach in drug regulatory procedures (Pappalardo et al., 2019; Zhuang & Lu, 2016). Advancing toward establishing models of nutrient digestion and absorption could help predict the metabolic responses to nutritional compounds, diet, and nutritional interventions (Le Feunteun et al., 2020).
Computational tools such as DIANA-mirPathv3 software can enable a holistic integration of interconnected aspects, including nutritional components, metabolic pathways, and physiopathological processes, providing the foundation for hypotheses to plan experimental studies for novel therapies or interventions (Carotenuto et al., 2016; Vlachos et al., 2015).
Pirim and Dogan (2020) used in silico tools to identify the putative roles and possible implications of selected Xeno-miRNAs in human diseases. They found that 13 human genes were shared targets of the miRNA groups sorted by species and brought proof of correlations with several cancer types, specifically in colon adenocarcinoma by Ingenuity Pathway Analysis. miRNA functional enrichment analysis also emphasized the putative implications of the dietary miRNAs in cancer pathways. This study provided in silico evidence for the involvements of animal-derived dietary miRNAs in cancer-related pathways, bringing to light the need for future study design to explore the roles of dietary Xeno-miRNAs in cancer and nutritional interventions for cancer prevention and management.
The development of in silico models is vital to understand and predict the complex human host–microbiota interaction and environmental factors involved. Heyde and Ruder (2015) created a unique in silico tools of a living intestinal microbial community, engineered with synthetic biology, that interacts with a biomimetic, robotic host. By modeling and computationally mimicking engineered gene circuits in these microbiota communities, the authors replicated complex behaviors in the host (Heyde & Ruder, 2015).
Computational models integrated with high-throughput data (metagenomics data) of individual microbiotas from IBD—patients and healthy subjects, with genome-scale metabolic models, have been proposed for predicting personalized dietary treatments for Crohn’s disease (Bauer & Thiele, 2018).
The QSAR, a quantitative method used to describe the structure–activity relationship of compounds, has been widely employed in drug design, material science, and chemistry and is beginning to be used in nutritional science. For example, QSAR has been employed to discover and screen food-derived bioactive peptides (W. Bo et al., 2021; Song et al., 2023) and can be used to assess toxicity and functionalities of food ingredients, as well as in the development of food supplements (Kar et al., 2017).
ML and AI combined with human-derived toxicological big data could be valuable tools for food safety assessment (Coecke et al., 2022). A recent study proposes the development, and further training, of a read-across structure–activity relationship, using an ECHA database. The authors suggested that this model had better reproducibility than animal tests (Luechtefeld et al., 2018).
Digital twin (DT) technology is emerging as a transformative force in healthcare systems, revolutionizing the way patient care is delivered.
DT is a virtual replica of living or nonliving physical entities, which can be used to simulate their behavior and performance in real time. Functioning as a dynamic concept, DT embodies a virtual replica of human organs, tissues, cells, or microenvironments that continually adapts to real-time data variations and predicts corresponding future scenarios (Vallée, 2023).
DTs enable healthcare providers and researchers to gather and analyze a large amount of patient data from different sources, including electronic health records, wearables, and medical devices (Armeni et al., 2022), representing a great opportunity for nutritional science innovation.
The tool can correctly predict new independent data, including, for instance, hepatic glycogen and gluconeogenesis, and quantify personalized expected differences in outcome for any diet (Silfvergren et al., 2021).
By leveraging advanced analytics, real-time data integration, and virtual simulations, DTs allow for a holistic view of the patient, leading to personalized treatments or nutritional interventions, considering individual characteristics, real-time physiological data, and medical history. DT technology could predict the risk of nutrition-related diseases, for example, diabetes or cardiovascular conditions in a personalized manner, based on individual genetics, dietary habits, lifestyle, and other factors. This information could then be used to develop a personalized prevention plan, which could include changes in dietary habits, food supplements, and so forth.
One of the most promising applications of DTs in nutrition science is in the field of diabetes research. In 2019, Shamanna and his team published a study on the use of DTs to predict the progression of type 2 diabetes. The study used data from over 1000 patients, including their genetics, medical history, and lifestyle factors, including diet. The researchers then created personalized DTs for each patient, which were used to simulate the progression of their disease over time. The DTs were able to accurately predict the progression of diabetes in over 80% of cases, and they identified several key risk factors that were previously unknown. This information could be used to develop more effective prevention and treatment strategies for diabetes patients (Shamanna et al., 2021).
Wageningen University recently completed a DT project in metabolic health. The aim of the project called “Me, my diet and I” was to develop a personalized DT that can predict changes to an individual’s blood values such as glucose and triglycerides to provide dietary advice with the goal to reduce cardiometabolic disease risk (Knibbe et al., 2022).
The main limitation of computational models is that they are dependent on the data they are trained on or are called upon to analyze, their value depends on the quality of the data, and their performance will degrade if they are not regularly updated. Consequently, feeding and updating these models using novel, emerging, human-relevant data are crucial.
Some examples of possible applications of omics/multi-omics approaches and computational models to nutrition research, their main limitations and possible ways to address these limitations are summarized in Table 6.
| NAM | Examples of applications to nutrition research | Limitations | Possible ways to overcome limitations |
| Omics/multi-omics approaches (e.g., transcriptomics, metabolomics, nutrigenomics) |
– To define the molecular mechanisms underlying diet-related diseases pathogenesis – Defining genetic and environmental (dietary) risk factors – Patient stratification for treatment and response (C. Hu & Jia, 2021; Mars et al., 2020) – Finding biomarkers for dietary intake to facilitate human nutritional studies (Collins et al., 2019) – Disentangling the composition, diversity, and function of human microbiome (https://hmpdacc.org/) |
Integration of different omics datasets is a challenging task that relies heavily on data mining and machine learning (ML) algorithms | Establishing committed consortia with a multi-disciplinary approach (e.g., joining molecular biology and bioinformatics expertise (Krassowski et al., 2020) |
| Missing data: Due to either cost, instrument sensitivity, or other experimental factors, data for a biological sample may be missing for one or more omic layers | Recent methodological developments in artificial intelligence (AI) and statistical learning have greatly facilitated the analyses of multi-omics data (Flores et al., 2023) | ||
| Computational models |
– Modeling molecular interactions, signaling and metabolic pathways – To support complex in vitro models for system-level understanding of complex processes – In vitro to in vivo extrapolations – To analyze/integrate multi-dimensional datasets – Analyzing the relationship between nutrients, human microbiome, health, and disease (Allison et al., 2015; Verma et al., 2016) – Modeling the digestion and absorption of several drug molecules and/or nutrients – “In silico clinical trials” for regulatory procedures (Pappalardo et al., 2019; Zhuang & Lu, 2016) – To predict the metabolic responses to nutritional compounds, diet, and nutritional interventions (Le Feunteun et al., 2020) – QSAR: To understand and predict complex human host–microbiota interactions (Heyde & Ruder, 2015); discovering and screening of food-derived bioactive peptides (W. Bo et al., 2021; Song et al., 2023); assessing toxicity and functionalities of food ingredients – To develop food supplements (Kar et al., 2017) – ML and AI: food safety assessment (Coecke et al., 2022) – Digital twins: food safety assessment, personalized nutrition, disease prevention |
Results based completely on existing knowledge and input data | Updating models using novel, emerging, human-relevant clinical and preclinical data |
| Performance impaired by low-quality data |
- Abbreviation: QSAR, quantitative structure-activity relationships.
3.6 AOPs
The science of toxicology and risk assessment is currently undergoing a paradigm change moving away from the use of apical toxicity endpoint in animal models, such as organ pathology, to an approach that is more reliant on understanding the mechanism of action underlying the adverse outcome (AO) in humans (National Research Council, 2007). This has given rise to the concept of AOPs, which is a linear sequence of events, including a molecular initiating event (MIE) induced by a stressor that interacts with a molecular target, followed by intermediate key events (KEs) at the cellular level, and eventually resulting in an AO at an organism or population level (Ankley et al., 2010).
The development of AOPs facilitates the gathering of mechanistic information in an organized way, to help establish fundamental relations between the molecular/cellular events that lead to adverse effects and allow to identify critical data gaps in understanding these pathways.
As proposed by Blaauboer et al. (2016), these new toxicological approaches can be applied to foods and food ingredients, providing an opportunity for integrating data from studies on food substances, from novel advanced in vitro systems, in silico, and human studies, and for developing a mechanistic understanding that can be applied to risk assessment. These approaches should be used that provide relevant information for the mechanism of action in humans.
The development of AOP networks has been suggested not only to enhance understanding but also to predict AOs in the field of risk assessment, research, and regulatory decision-making (Knapen et al., 2018; Villeneuve et al., 2018).
Since consumers are increasingly exposed to novel proteins or protein-containing products, the AOP framework could be useful to support a comprehensive and human-relevant risk assessment complying with the European “Novel Food” law, for example, to assist in the prediction of the risk for food allergy development. This could be done by harnessing the considerable body of (human) in vivo and in vitro data describing molecular and cellular events potentially involved in food sensitization (van Bilsen et al., 2017).
Importantly, the AOP concept has been more recently recommended as a tool for mapping the perturbation of normal human physiology during disease development (Langley et al., 2017; Marshall et al., 2018). In particular it has already suggested for the description of critical endpoints in the human pathogenesis of COVID-19 (CIAO-Covid, 2020) and Alzheimer’s disease (Tsamou et al., 2021).
A disease AOP, similarly as AOP in toxicology, describes a sequence of causally related KEs triggering downstream effects at different biological levels and provides strong mechanistic bases for preventive, diagnostic, and therapeutic interventions. The key steps are similar, although the MIEs may be different. For example, analogously as for chemical perturbations, food and/or microbiota-related factors may trigger a disease process. Through an AOP conceptual framework, it could be possible to congregate existing knowledge about signaling pathways that are perturbed during the different stages of a specific disease and to link genetic determinants and lifestyle factors, including dietary habits, with adverse health effects (Pistollato et al., 2015).
The AOPs framework, fed by the ever-increasing amount of human-relevant data coming from novel in vitro and in silico tools, as well as in vivo human studies, represents a great opportunity to support food safety assessment processes and to advance biomedical research, including nutrition research.
4 DISCUSSION
It is now clear that traditional animal and cell culture models may not be reliable for studying complex pathophysiological aspects of human diet-related diseases, for designing effective therapeutic interventions or for food safety assessment. This deep gap in translational research highlights the necessity for a paradigm change in nutrition research, from animal models and suboptimal in vitro cell systems, toward a more reliable and reproducible human conceptual framework.
Importantly, opportunities for using NAMs have been highlighted in recent European Food Safety Authority (EFSA) risk assessment guidelines. Moreover, EFSA’s 2027 Strategy encourages a more regular reliance on NAMs in support of food safety assessments, recognizing the need for paradigm evolution and guidance from apical effects measured in animal studies to use NAM-based results in food risk assessment (Cattaneo et al., 2023).
Additionally, the introduction of novel protein sources into the human diet, including recently developed original foods or foods produced by means of new production processes or technologies, (e.g., algae, insects, duckweed, creates the opportunity for the development of new food-associated health risks, and this in turn is creating the necessity to develop and apply human-relevant test methods suitable for characterizing the allergenic potential of novel foods.
Here, we have detailed some of the most promising nonanimal NAMs that can be leveraged to explore cellular and molecular mechanisms related to dietary-associated diseases, nutraceuticals, effective preventive strategies, and food risk assessment, toward a more human-based conceptual framework, as already happening in toxicology and regulatory testing (Fischer et al., 2020; Krewski et al., 2010). The envisioned human-oriented framework will not only improve human relevance and translatability but also contribute to the reduction/replacement of animals traditionally employed in nutrition research. Currently, in Europe, about 45,000 animals per year are used only for regulatory testing in the food sector (de Boer et al., 2020). In this epoch of growing concern for the ethical justification of the use of animals in research, it is vital to consider not only the methodological dimensions but also the ethical implications of the use of sentient beings in nutrition research, in line with the Directive 2010/63/EU on the protection of animals used for scientific purposes, whose ultimate aim is to replace all animal research with nonanimal methods (EuropeanParliament, 2010, Recital 10).
On December 2022, the FDA Modernization Act 2.0 was signed into law. The law fundamentally negates the Federal Food, Drug, and Cosmetics Act of 1938, which mandated animal testing for every new drug development protocol. Since this bill allows companies to submit nonanimal data employing alternative methods to demonstrate the safety and efficacy of investigational drugs prior to human trials, it is expected that it will facilitate the adoption of nonanimal NAMs (Han, 2023).
However, one of the biggest barriers to extensive NAMs adoption seems to be associated with biases, for example, most scientists who submit a grant application or paper based on in vitro findings usually expect to find at least one reviewer who asks that supplementary animal experiments be performed to validate their results before the work could be suitable for publication or funding (Ingber, 2020). The so called “animal-methods bias” in scientific publishing is a recently defined kind of publishing bias describing a preference for animal-focused methods where nonanimal human-based NAMs may already be suitable or where they may not be necessary, which influences the likelihood or timeliness of a manuscript being accepted for publication (Krebs et al., 2022).
As described by Ingber (2020), “the problem mainly lies in the fact that many animal models are physiologically irrelevant when considering human disease, and thus, demanding use of a poor animal model for the sole sake of satisfying Reviewers should be discouraged.” The author suggested that given recent advances in OoCs technologies, these innovative tools provide more physiologically and clinically reliable in vitro preclinical models for assessing both physiopathology and pharmacological responses than many animal studies (Ingber, 2020).
However, it is important to note that OoCs, iPSCs-derived human organoids, and other sophisticated cellular models—while addressing human relevance—would still constitute the lower scale/level of “wet lab” research. Therefore, computational approaches together with global epidemiological datasets represent the key tools necessary to account for higher scale/level and to determine systemic correlations among diet, health, and disease. In this regard, it should be considered that the progress in NAMs, including in vivo imaging technologies, omics approaches, and the discovery of new biomarkers of dietary intake, are increasingly facilitating human in vivo studies (McNamara & Brennan, 2020; Senekowitsch et al., 2022).
The feasibility of the envisioned human-oriented strategy in nutrition research necessarily requires the integrated application of different NAMs, approaches, and readouts, accounting for different levels of biological complexity, from molecular/gene level to organism/population scale (Figure 6).

This consequentially requires several fields of expertise and laboratory facilities: The creation of a collaborative scenario is needed to determine the complex relations between human health and diet and for food safety assessment.
Clearly, we recognize that some forms of animal testing will be probably carried out for many years to come, in order to cross-validate NAMs results with human data and to gradually persuade animal researchers and regulatory scientists of their value.
Obviously, currently, no NAM taken individually constitutes an ideal model, and we know that a perfect model does not exist; however, using NAMs in an integrated or tiered way (Andersen et al., 2019) and in combination with human observational and intervention studies would greatly benefit nutrition research. It should also be considered that while we have seen a rapid evolution of NAMs, in a manner incomparable to that of animal models, and that NAMs have great development potential, it has been suggested that animal models can be improved only to a limited extent, and they can never be completely externally valid due to the uncertainty introduced by animal–human species differences (Pound & Ritskes-Hoitinga, 2018). It is not possible to change the biology of the mouse, which however humanized and perfected will always remain a mouse.
5 CONCLUDING REMARKS
Human stem-cell in vitro models, OoC and MPS, high-throughput “omics” readouts, in silico/computational models, together with data obtained from meta-analysis of observational and interventional studies, and the description of AOPs, are among the ideal tools to elucidate the complex relations between nutrition and human health and disease, accounting for multiple levels of complexity, from population/individual level down to molecular level. The extensive adoption and further development of human-based NAMs would allow a better understanding of human nutritional pathophysiology and the development of more effective targeted therapeutic or preventive interventions while helping in reducing the number and replacing animals employed in nutrition research and food safety risk assessment. Since consumers are increasingly exposed to novel food products, these tools and approaches may prove particularly useful to support research in this emerging field.